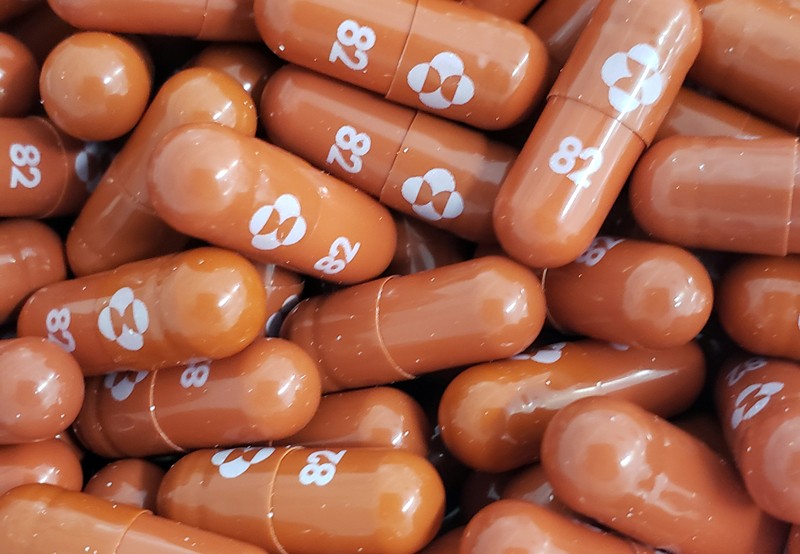
MANILA, Philippines – RiteMed Philippines Inc (RiteMed), in partnership with Faberco Life Sciences Inc., (Faberco), announced that the first batch of anti-Covid oral drug Molnupiravir, under the brand name MOLNARZ®, has arrived in the country, allowing the company to start its distribution initially to hospitals that have secured compassionate special permits (CSP) from the Food and Drug Administration (FDA).
RiteMed President Jose Maria A. Ochave said depending on the issuance of necessary approvals and conditions from the FDA and the Department of Health (DOH) they are also hoping to make Molnupiravir more accessible to Filipinos via the drugstores.
“It is our hope that Molnupiravir will also be cleared for distribution to drugstores nationwide soon so we can make it readily accessible to Filipinos, which is in line with RiteMed’s longtime advocacy of giving the public better access to quality affordable medicines. It can be used to treat mild and moderate symptoms at home, but we need to make sure that its use is subject to supervision by doctors and comply with the requirements laid down by FDA,” Ochave said.
Right now, Molnupiravir, the first oral drug clinically proven to reduce the risk of hospitalization and death from COVID-19 by 50 percent, will only be made available in hospitals that were given CSPs by the FDA nationwide.
Molnupiravir, developed by Merck & Co. (known outside the US and Canada as MSD), prevents further replication of the SARS-CoV-2 virus by targeting its ribonucleic acid (RNA) polymerase — an RNA-dependent enzyme.
RiteMed is distributing MOLNARZ® (molnupiravir) 200 mg capsule together with Faberco, which was appointed by Aurobindo Pharma Ltd., a licensed manufacturer of Merck & Co, to import the anti-Covid pill to the country. RiteMed and Faberco have agreed to a strategic partnership to help provide quality and affordable treatment solutions not only to COVID-19 but also to other diseases that impact on public health.
RiteMed will distribute MOLNARZ® (molnupiravir) to hospitals, medical institutions, and treatment sites under the CSP , which allows experimental drugs and vaccines to be prescribed by doctors and administered to patients as long as their use would be monitored for potential adverse reactions.
Faberco has initiated a rolling evaluation to the FDA for Emergency Use Authorization (EUA) for Molnupiravir. This comes after the United Kingdom granted an EUA to Molnupiravir. Further distribution of this life-saving drug to drugstores will be subject to the approval and conditions set by the FDA.Once the EUA is issued, Faberco will handle all local government unit orders while RiteMed will handle all the other channels, including hospitals and drugstores.
Patients discovered with mild to moderate Covid will need to take the pill for five days to prevent the disease from progressing. Clinical studies have shown that it is effective against Delta, Beta, and other variants.